Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
You can’t start a company without a healthy dose of courage, and this is certainly the case Neurobionics. The MIT-spinout thinks it could one day improve the lives of millions of people living with neurological conditions like depression, epilepsy and Parkinson’s disease.
If all goes well for the 18-month-old garment, his approach could further address “the peripheral nervous system for pain, incontinence and a number of other applications,” said Steve Jurvetson, a celebrity investor at Future Ventures.
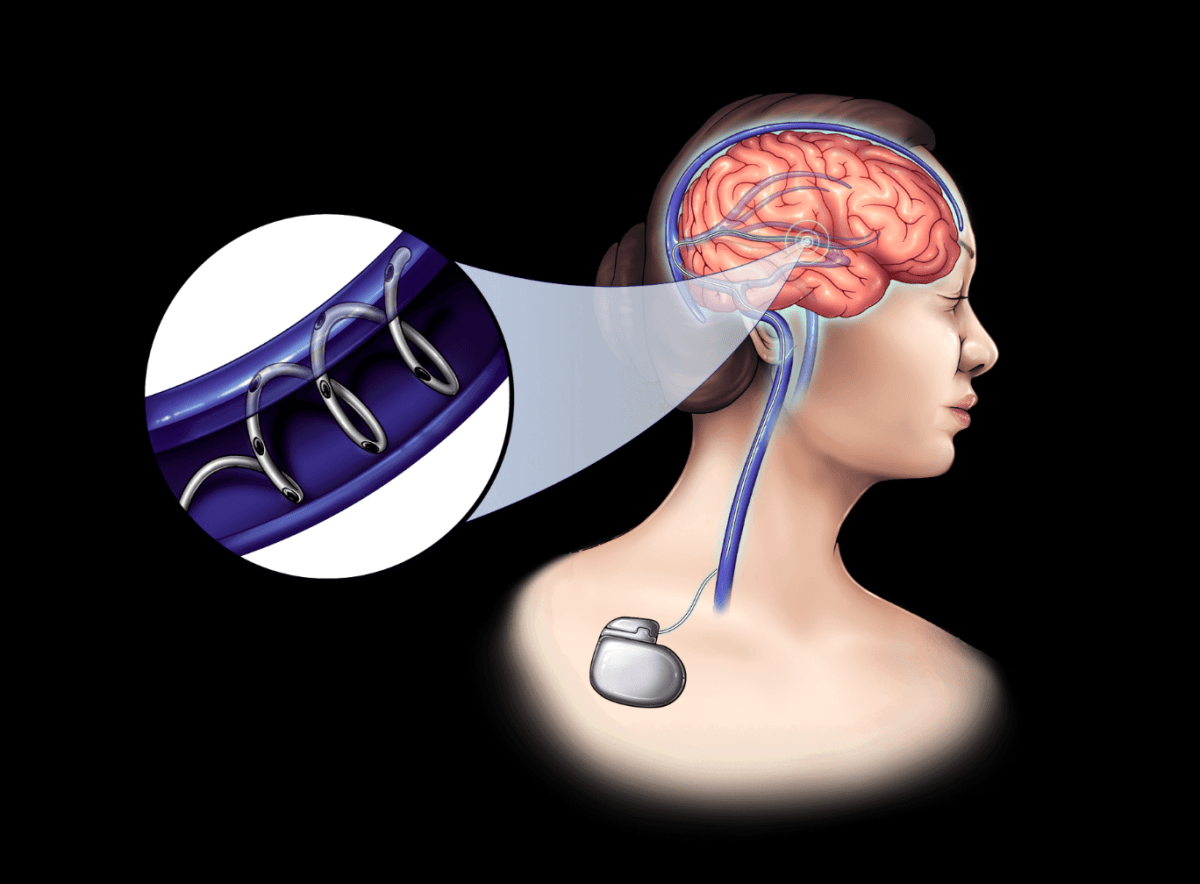
How? With what? In contrast to these big ambitions, NeuroBionics technology is small. Specifically, NeuroBionics aims to thread what it’s developing—bioelectric fibers the width of a human hair—through blood vessels in the brain using a procedure similar to stent placement to deliver neuromodulation therapy.
The fibers are powered by a fairly standard implantable battery, similar to an Airpod case, that will last five to 10 years and is used by other medical device manufacturers for spinal cord stimulation.
It’s a pretty cool alternative to drilling into someone’s skull, as has long been the case with deep brain stimulation. Traditionally, when certain disorders do not respond to medication, metal electrodes are implanted in the brain to generate electrical impulses and control this abnormal movement.
NeuroBionics’ device is no less invasive—the company uses carbon nanotubes instead of thin-film platinum or iridium oxide, which are common materials for these electrodes. Although metals are minimally toxic and conduct electricity well, they can also melt, limiting lifespan and causing tissue damage. Carbon nanotubes, on the other hand, are cheaper, can last longer, and make MRIs much easier to obtain. (Among other things, metal can cause bright spots on MRI images, making it difficult to see the brain.)
The whole shebang is the result of 10 years of research into fiber technology at MIT, according to MJ Antonini, CEO of the Cambridge, Massachusetts-based startup. He co-founded the company while a student at the school, where he secured three patents that gave MIT a small stake in the business.
He took an interesting route from point A to point B. While demonstrating a twisted version of the elusive fiber, Antonini explained that the 55-year-old holds PhDs from Harvard and MIT. program called the Harvard-MIT Program in Health Sciences and Technology.
Calling it “a niche program that they don’t advertise for the wrong reasons,” Antonini said his education included two years of medical school at Harvard, followed by medical engineering and medical physics at MIT. After that, he decided to “move beyond the cool (research) paper” and “create an actual product and an actual medical company.”
Indeed, Antonini, who is French, said he is staying on as a postdoctoral researcher for a few more years to think about how he can bring this technology portfolio to the real world. Finally, in early 2023, he dropped out of school with Nicky Driscoll, a postdoctoral researcher at MIT and today the CTO of NeuroBionics.
It will take a long time to know what their fiber technology will be. Like Jurvetson, Antonini insists that eventually NeuroBionics’ bioelectronic fibers could be used in a whole range of applications, including drug delivery, cutting tissue in the brain, and treating conditions related to the spinal cord and peripheral nervous system.
“Finally” is still a while away. So far, the outfit has closed $5 million in funding led by Dolby Family Ventures, with participation from Future Ventures, GreyMatter Capital and several other backers, and will use the capital to complete work on its clinical device.
Once complete, the next step will be to try to demonstrate its safety and efficacy in pigs, which have many similarities to humans in terms of anatomy, physiology and genetics. The FDA will then review this case, after which NeuroBionics can apply for an investigational device exemption (IDE). It can then run the first feasibility studies in humans.
When asked when the technology could hit the market, Antonini hesitated for a moment before suggesting 2030.
Of course, he wouldn’t have worked on it if he didn’t think the startup could handle those next steps.
Sick investors like Jurvetson need help. “Deep brain stimulation has been shown to work for stroke, epilepsy, Parkinson’s, Alzheimer’s, chronic pain, tremors, and more.” Jurvetson wrote in an email: “But 99% of people who could benefit are rightly turned away because it requires major open brain surgery with needles implanted deep in the brain.”
As far as Jurvetson is concerned, technology like NeuroBionics opens that market wide — including to a concentrated pocket of large and advanced hospitals that offer the surgery today.
The “field of application” for the startup’s “minimally invasive stent” is huge, Jurvetson said.