Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124

The food and medication administration has classified a retirement of 60 products of different donuts to its second highest, the agency announced earlier this week in an update of its website.
The retirement was announced for the first time on January 7, but was classified by the FDA as a “class II retreat” on February 5.
The retired products were produced by FGF Brands, and were sold in groceries throughout the United States and Canada, said the FDA report on retirement. The total retirement amounts to more than 2 million cases of donutsBiñones, Paczki, Eclairs and Munchkins.
The FDA remembers breadcrumbs throughout the country
All products occurred before December 13, 2024, and are “within the expiration,” says the FDA compliance report.
Listeria monocytogenes, the bacteria that may have contaminated The donutsIt can cause Listeriosis if consumed, said the website for centers for disease control and prevention.

Doned withdrawn (not in the photo) may have been contaminated with Listeria, said the FDA. (Istock)
“It is estimated that 1,600 people get Listeriosis every year, and around 260 die,” said the CDC.
Those who have the greatest risk of Listeria are “pregnant women and their newborns, adults of 65 years or older, and people with weakened immune systems,” said the agency. Other groups “rarely get seriously ill” when they are exposed to Listeria, the CDC pointed out.
Click here to register in our lifestyle bulletin
Not born babies have a particular risk of Listeria, said the CDC.
“The infection during pregnancy results in fetal loss in approximately 20% of cases and the death of the newborn in approximately 3% of cases,” said the agency.
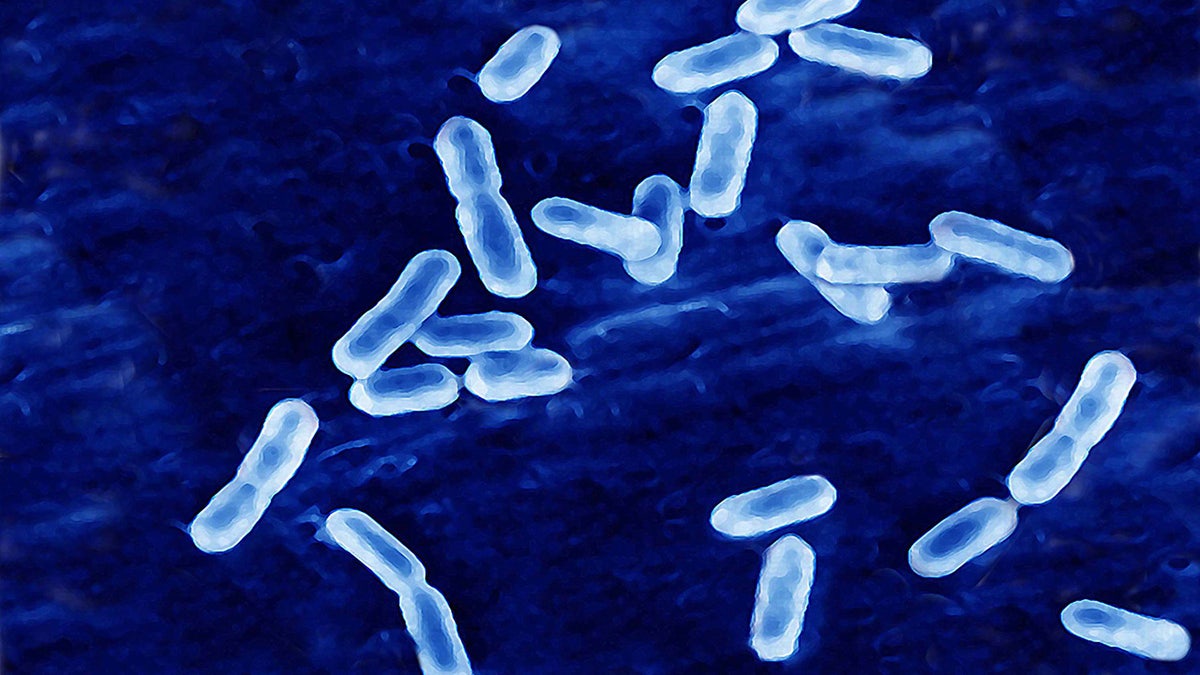
Listeria Monocytogenes (above) can cause Listeriosis, which can be deadly for certain groups. (Bsip / UIG through forty images)
A 2024 Listeria outbreak Connected with a Delicatessen meat processing plant killed nine people and resulted in dozens of hospitalizations, Fox Business previously reported.
A class II retreat is the second highest classification of the FDA retreats, says its website.
For more lifestyle, visit www.foxnews.com/lifestyle
These are issued when there is “a situation in which the use or exposure to a violation product can cause adverse consequences of temporary or medically reversible adverse health or when the probability of adverse consequences of serious health is remote,” says the site FDA website.

The FDA classified Donas withdrawal on February 5. (Reuters/Andrew Kelly/File Photo)
This contrasts with a memory of “Class I”, the highest level.
Click here to get the Fox News application
TO Class I record It is for “a situation in which there is a reasonable probability that the use or exposure to a violator product causes serious adverse consequences for health or death,” according to FDA.