Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
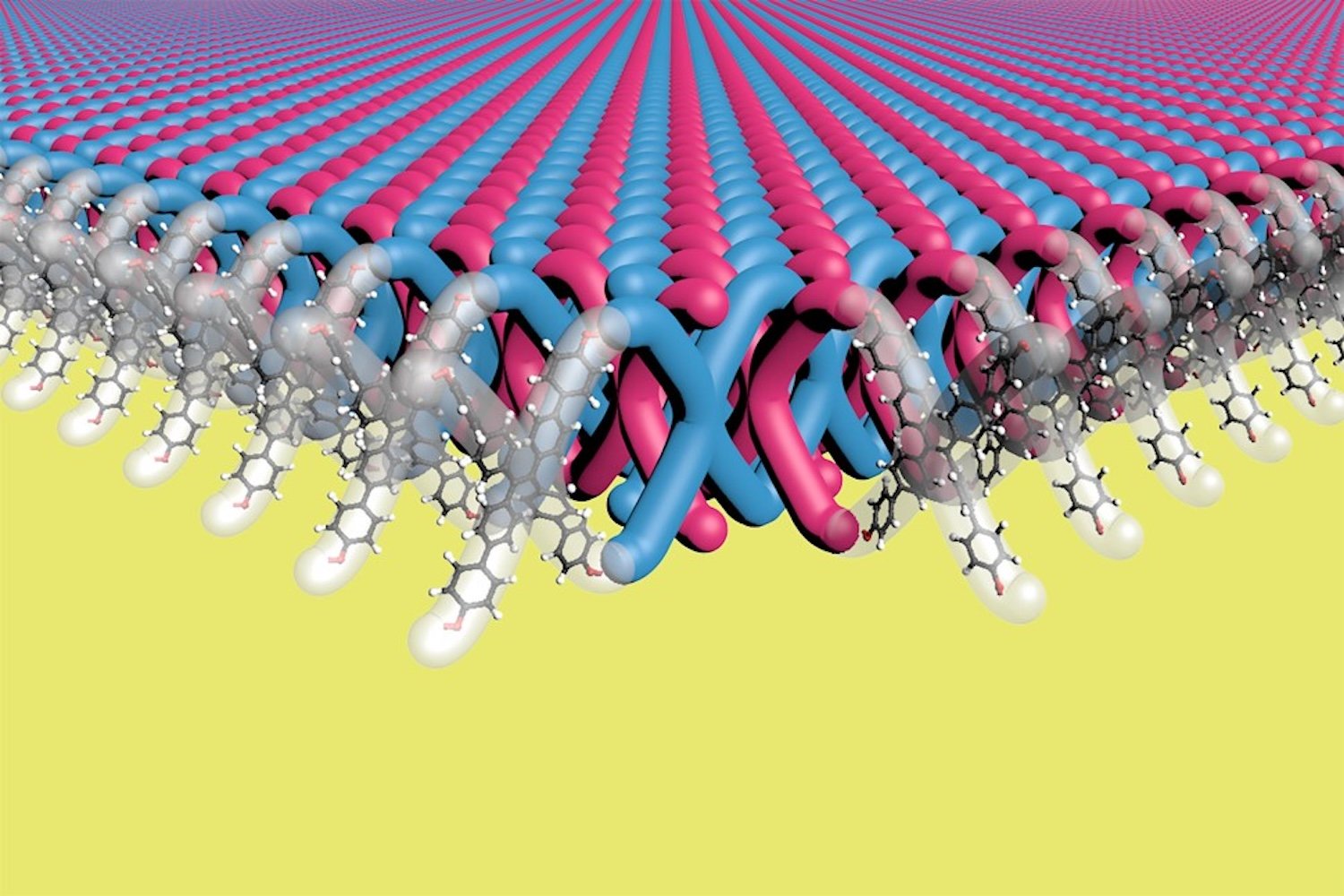
Imagine armor made of materials as light as cloth but stronger than steel, linked together like molecular chains. Scientists may have taken the first step towards making this a reality.
A team of researchers led by Northwestern University scientists has developed the first two-dimensional (2D) mechanically interlocked material, similar to links in chain mail. Detailed material on January 16 to learn published in the magazine Scienceextremely flexible and strong, with promising applications in products such as lightweight body armor and ballistic fabrics.
The researchers built the material at the nanoscale level, meaning its individual components can be measured in nanometers. Technically a polymer: a substance composed of large molecules composed of smaller chemical units called monomers. Examples of polymers include proteins, cellulose, and nucleic acids.
A 2D mechanically bonded material is a polymer structure that utilizes mechanical bonds – i.e. physically bonded bonds – as opposed to the covalent bonds that typically make up polymers and involve the sharing of electrons. According to the researchers, the material contains 100 trillion mechanical bonds per 0.16 square inches (1 square centimeter), the highest density of mechanical bonds ever created.
“We created a completely new polymer structure,” said study co-author William Dichtel of Northwestern University. statement. “It’s like chain mail in that it can’t break easily because each of the mechanical links has some freedom to slide around. If you pull it, it can disperse the applied force in several directions. If you want to break it, you’re going to have to break it in many, many different places. We continue to study its properties and will probably learn for years.”
The biggest challenge in creating mechanically linked molecules is how to orient the polymers to form mechanical bonds. Madison Bardot of Northwestern University, who led the study, is tasked with finding a new way to achieve this. The team placed the x-shaped monomers into a crystal structure (a special ordered arrangement) and reacted the crystals with another molecule. This reaction created mechanical bonds in the crystals. The end product is 2D layers of interconnected polymer layers made from these bonds between X-shaped monomers, which fill the researchers’ voids with more X-shaped monomers.
“This was a high-risk, high-reward idea where we had to question our assumptions about what kinds of reactions are possible in molecular crystals,” Dichtel said. The resulting material is incredibly strong, but still flexible and easy to manipulate, because when the polymer is dissolved in a solvent, the individual layers of interconnected molecules separate from each other.
“Once the polymer is formed, there’s not much that holds the structure together,” he said. “So when we put it in a solvent, the crystal melts, but each 2D layer stays together. We can manipulate those individual sheets.”
While previous researchers have made very small amounts of mechanically bonded polymers that would be difficult to mass-produce, the team’s new method is surprisingly scalable. They made more than a pound (0.5 kilogram) of material and offered the ability to produce more.
However, even a small percentage of the new polymer structure can improve other substances. The researchers developed a material consisting of 97.5% Ultem fiber (an extremely stiff material in the same family as Kevlar) and 2.5% 2D polymer, and concluded that the mixture significantly strengthened the former.
“We have more analysis to do, but we can say that it increases the strength of these composite materials,” Dichtel said. “Almost every property we’ve measured has been exceptional in some way.”
This incredibly strong and flexible material could be the armor of the future.