Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
World BBC service
 BBC
BBCThe Indian authorities have prohibited two highly addictive opioids in response to a BBC investigation that discovered that they were feeding a public health crisis in parts of Western Africa.
In a letter seen by the BBC of the general controller of the drug controller of India, Dr. Cajeev Singh Raguvanshi said that permission to manufacture and export drugs had retired
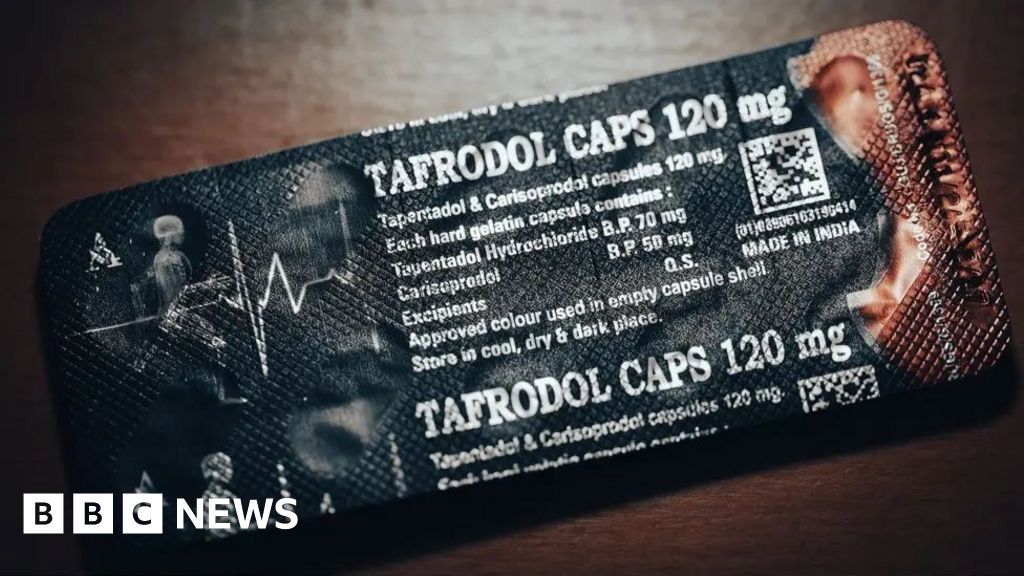
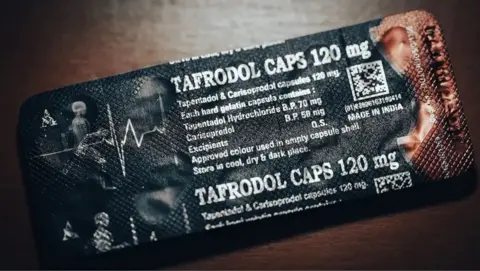
BBC Eye found a pharmaceutical company, Aveo, had been illegally exporting A harmful mixture of Tapentadol and Carisoprodol in countries such as Ghana, Nigeria and Ivory Coast.
The Food and Medicines Administration of India said that the company’s factory in Mumbai had been raided and its complete action seized.
Dr. Raguvanshi’s circular, dated until Friday, cited the BBC investigation in his decision to ban all the combinations of Tapentadol and Carisoprodol, which should be implemented with immediate effect.
He said that this also occurred after the authorities had examined “the potential of drug abuse and their harmful impact on the population.”
Tapentadol is a powerful opioid, and Carisoprodol is a muscular relaxant so addictive that it is prohibited in Europe.
Carisoprodol is approved for use in the US, but only for short periods of up to three weeks. Abstinence symptoms include anxiety, insomnia and hallucinations.
The combination of the two drugs has no license for use anywhere in the world, since they can cause respiratory difficulties and seizures and an overdose can kill.
Despite the risks, these opioids are popular street drugs in many western Africa countries, because they are very cheap and widely available.

The publicly available export data show that Aveo Pharmaceuticals, along with a sister company called Westfin International, has sent millions of these tablets to Ghana and other Western Africa countries.
The BBC World Service also found packages of these pills with the Aveo logo for sale in the streets of Nigeria and in the cities and cities of Ivairian.
Nigeria, with a population of 225 million people, provides the largest market for these pills. It has been estimated that around four million Nigerians abuse some form of opioids, according to the National Office of Statistics of the Nation.
As part of the investigation, the BBC also sent an undercover operation, which makes an African businessman through Sharma, showing the same dangerous. Products The BBC found for sale in West Africa.
In the images recorded in secret, the operation tells Sharma that his plan is to sell the pills to adolescents in Nigeria “who love this product.”
Sharma in response responds “OK”, before explaining that if users take two or three pills at the same time, they can “relax” and accept that they can reach “high.”
Towards the end of the meeting, Sharma says: “This is very harmful to health,” and adds that “today, this is a business.”
Sharma and Aveo Pharmaceuticals did not respond to a request for comments when the BBC’s initial investigation was published.
The Food and Medicines Administration of India said that a sting operation saw the entire Aveo action seized and a greater production stopped in a statement on Friday. More legal measures will be taken against the company, he added.
The agency said it was “completely prepared” to take measures against anyone involved in “illegal activities that tarnish the country’s reputation.”
The FDA has received instructions to carry out more inspections to prevent the supply of medicines, he said.